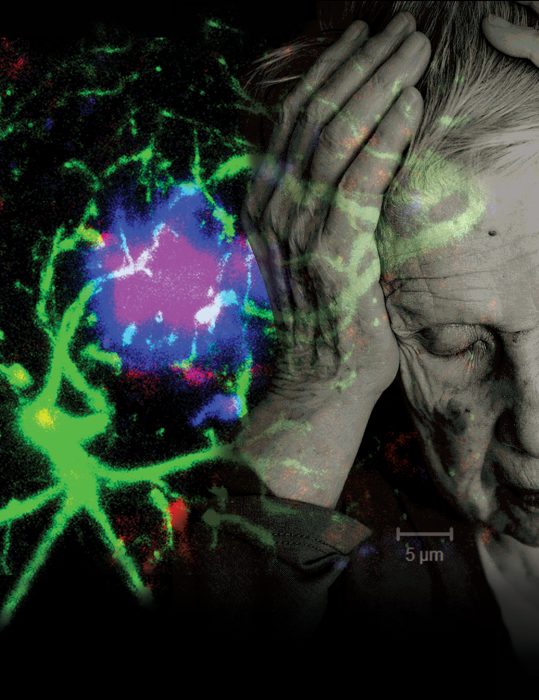
ALZHEIMER’S
Dr. Erhard Bieberich has found that ceramide’s positive role in the brain’s early development can turn ugly in cases of Alzheimer’s disease.
It is a lipid likely best known for improving the look of our skin and hair.
However, the ceramide studies of Dr. Erhard Bieberich are all about form and function. In a decade of work, the Medical College of Georgia neuroscientist has helped define how this waxy molecule plays a widely divergent role in our brains from healthy formation to destruction in Alzheimer’s disease.
Early on, ceramide helps stem cells line up properly to form the primitive ectoderm from which the embryo develops, Bieberich along with collaborator Dr. Guanghu Wang, MCG stem cell biologist, and others reported in 2007. Bieberich would theorize then that ceramide aided cell polarity – literally deciding which end is up – so a wad of cells could ultimately form a complex fetus.
But ceramide’s directing has only just begun. It helps ensure that the antennae or cilia — the eyes of cells that sense the environment and tell cells where they need to go and what they should do when they arrive — stay up.
“A cell is blind; it does not see; it does not feel; it does not know where it is,” says Bieberich. Ceramide and the antenna help cells gain a sense of perspective and direction, so as they start moving out to form a fetus, brain cells for example don’t wander off to the feet. The scientists would find ceramide keeps the antenna up by inhibiting the enzyme histone deacetylase 6, or HDAC6. It seems cells can’t divide and do much else, so antennae retract and HDAC6 gets activated when they need to divide.
At this early point, ceramide seems just good for the brain. Because as the brain now begins to form, stem cells divide too much, resulting in a surplus of cells that could readily form a tumor. Bieberich’s group found that a short version of the typically long ceramide also shows up to help kill off these cells. His work with Dr. Brian Condie at the University of Georgia showed that the ceramide partners with another protein, PAR-4, to kill off these potentially harmful cells.
His team would also find parallels in Alzheimer’s: that retracted antennae is another sign of the age-related disease; that the short version of ceramide also coats the dysfunctional antennae of some brain cells in Alzheimer’s; and that the lipid-protein package that helps destroy excess stem cells during development also kills adult brain cells we need to keep.
 When ceramide goes bad
When ceramide goes bad
Astrocytes are star-shaped brain cells that normally nurture neurons. Like a mobile gas station, they pull up to active neurons and fill them with plenty of blood, oxygen, glucose and other essentials, since these cells – that connect to each other and our brain to our body – do not have a direct blood supply. But when their antennae inexplicably retract, astrocytes shift their attention to themselves and to dividing. It seems these direction-finders should be coated with the long version of ceramide, which helps regulate their growth. But wayward astrocytes are coated with the same shorter ceramide that helped kill off stem cells during development.
“If an astrocyte is dividing, you can imagine it uses all the fuel up for itself,” Bieberich says. In reality, they can’t divide and nourish simultaneously because they need the same molecular machinery to divide and to keep their cilia full staff. To worsen matters, the now dividing astrocytes become inflammatory, shedding inflammation-producing immune factors called cytokines along with growth factors that also inflame. He has evidence that when inflammation is present, cilia are not.
“If you looked at a developing brain or the brain of a patient with Alzheimer’s or even cancer, the cilia would be [lowered] because cell division is going on,” Bieberich says. “In a healthy, mature brain, you need to have sensory organelles like cilia turning growth factors off and instead finding the best position around the neuron to be nurturing.”
As the dysfunctional relationship escalates, neurons start secreting more amyloid, essentially misfolded proteins with a propensity to stick together. The hallmark beta-amyloid is basically little snippets of the precursor of this protein. Amyloid precursor protein is found in many places but is concentrated in the synapses of neurons, where some believe it is involved in the formation of these connections between neurons as well as the adaptivity or plasticity of brain cells. However, as these snippets increase in number and congregate into plaques, they appear to do just the opposite: They interfere with individual cell communication and overall brain function.
Consistent pragmatist Bieberich sums it up: “If the neuron makes something toxic and dumps it at your door, what would you do?”
One thing astrocytes do again is reach into their developmental past, to team ceramide with PAR-4. Both can do independent damage, but together, they become a sort of suicide pill for neurons.
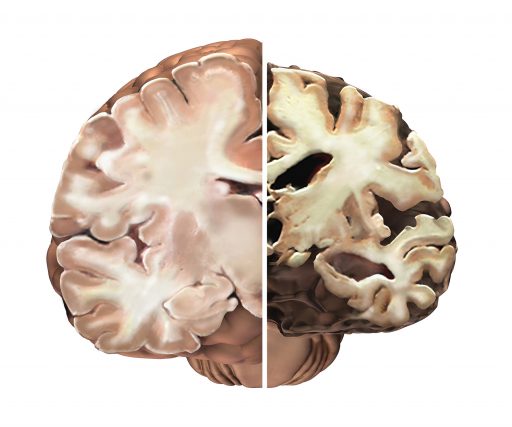
More typically, ceramide and PAR-4 aren’t anywhere near each other, rather in distinct areas of a cell. But when faced with amyloid, ceramide appears to take the lead in bringing the two together as they were in the developing brain. The scenario gets more bleak as the pairing may result in a sort of murder-suicide as the deadly package ultimately takes out the astrocytes as well. Of course, neurons can’t survive without astrocytes, so a wave of brain-cell loss can follow. This murder-suicide scenario helps explain the death and literal brain size shrinkage seen in Alzheimer’s. “It’s kind of a contextual lipid: It can be good or bad,” Bieberich says.
But there also is potential for intervention as those studies published in 2012 showed that, in the brain cells from humans with Alzheimer’s as well as an animal model, antibodies to ceramide and PAR-4 stopped the carnage.
Can you make it good again?
His newest two grants totaling $4 million from the National Institutes of Health are enabling Bieberich’s pursuit of the different sets of proteins he believes long and short ceramide bind to. His team’s ultimate goal is restoring the good ceramide and preventing the problematic cascade that results from its shorter counterpart. At the very least, he wants to help turn down the bad ceramide and slow down disease.
“We think by manipulating ceramide metabolism — creating the right ceramide so to speak — we can reinstall ciliogenesis. We can convince the cell to return to the quiescent state,” he says.
But it’s not just about the right kind of ceramide, but the right amount. Too much ceramide has long been associated with cell death, and ceramide levels are known to be elevated in the blood of patients with Alzheimer’s. It can be found in their brains on autopsy, and Bieberich has seen it in the plaques of his animal model.
In what becomes a vicious cycle, rather than helping out brain cells like in the earliest days of life, high levels of ceramide appear to increase beta-amyloid production, which, in turn, further increases ceramide production. In another biological irony, his team also has documented increased levels of ceramide antibody in their Alzheimer’s model. But rather than fight the disease as antibodies are want to do, they support disease, particularly in their female mouse model. The surprising work, published in 2015 in the Journal of Alzheimer’s Disease, gave more legs to the concept that Alzheimer’s is an autoimmune disease, noting that this attack on self-tissue by our immune system is generally more common in women.
About Ceramide
The insulation on our nerve cells is made of ceramide derivatives. Our cells make ceramide, and we consume it in plant foods and meats; but because of its potential toxic effect, levels tend to remain low. However, in the case of cancer, it may be the more ceramide the better, as scientists are looking at ways to selectively elevate it to destroy some cancers, while Bieberich and others would love to turn it down in Alzheimer’s. About 50 percent of our brain is lipids, although Bieberich refuses to laugh when asked if that is scientific proof that we are all fatheads. Fat is a kind of lipid, not the other way around, he explains mostly patiently. The kind we have in our heads is definitely not what collects in our bellies.
It takes a while for the com-pounding scenario to turn toxic, since there’s a natural clearance system. But at some point the system simply cannot keep up, so amyloid and ceramide pile up. The researchers tried to help the natural process by injecting even more ceramide under the skin where it should trigger an immune response that slows disease progression.
“We thought, we can immunize the mouse against its own ceramide; it develops antibodies, which neutralize the ceramide; and we get a similar effect as blocking its production, like a vaccine against it,” Bieberich says. That’s when they realized that antibody levels were already high, apparently prompted by high ceramide levels that had worked their way into the bloodstream.
When given more ceramide, female Alzheimer’s mice were most affected, producing about 33 percent more amyloid. Production of exosomes – a sort of package astrocytes make to scoop up amyloid, which instead worsens this scenario – shot up 2.4 times. The gender difference, again, was not super surprising since two-thirds of Americans with Alzheimer’s are women, possibly because they tend to live longer, the Alzheimer’s Association says. And since Bieberich had already found that female mice have higher levels of antibodies in response to the higher levels of ceramide, levels of the lipid may well become a new hallmark of and possibly a biomarker for Alzheimer’s.
High ceramide in the brain also prompts astrocytes to produce these exosomes, or “lipid bubbles,” says postdoctoral fellow Dr. Michael B. Dinkins, the 2015 study’s first author. These bubbles can start piling up around brain cells; and the lab had found that when they are taken up by cells, the bubbles trigger cell death, which they reasoned is at least one way ceramide contributes to the brain cell destruction found in Alzheimer’s.
In a paper published this summer in The Journal of Neuroscience, the team reported that the exosomes astrocytes produce in response to neurons piling amyloid at their doorstep ultimately contributed to disease. In their animal model with several genetic mutations found in types of Alzheimer’s that tend to run in families, they showed exosomes piling up close to astrocytes, blocking the brain’s natural clearance system and further accelerating the production of beta-amyloid. In such volume and close proximity to neurons, exosomes begin to interfere with communication and nutrition, neurons stop functioning well and eventually begin to die, a scenario that again fits with disease progression.
They also removed some ceramide from the equation. One of their mouse groups was genetically programmed to make a nonfunctional form of the enzyme neutral sphingomyelinase-2. Amyloid activates this enzyme, which converts another lipid, called sphingomyelin, into ceramide. Their male mice, which were also sphingomyelinase-deficient, developed fewer plaques and exosomes, produced less ceramide and performed better in cognitive testing. It was the first evidence that brain cells that make fewer exosomes are somewhat protected. It is also an indicator that drugs that inhibit exosome secretion might be an effective Alzheimer’s therapy, Bieberich says.
“We show clearly that sphingomyelinase is causative here in making ceramide, making exosomes and in making plaques,” Bieberich says. He and his team already are testing different drugs given to patients for reasons other than Alzheimer’s that may also inhibit sphingomyelinase and ultimately ceramide and exosome production, at least in males.
For unclear reasons, the female mice again did not fare as well as the males, even with what should be an impaired ability to make ceramide.
“Atherosclerosis, metabolic problems, Alzheimer’s — a lot of things can happen to an older brain,” the neuroscientist says. He affirms that while all ultimately fit hand-in-hand, the fact that today these maladies cannot be treated effectively is the ultimate problem, but not the initial one.
“The initial problem is we don’t even know when it starts,” he says, because any cures or truly impactful therapies will have to come, in the case of Alzheimer’s for example, before plaques have encased neurons. And there could be plenty of lead time. The work of others has shown evidence of known Alzheimer’s players such as beta-amyloid in the blood of patients with familial Alzheimer’s disease as much as 10 years before symptoms surfaced.
Developing a diagnostic tool is definitely one of his next goals.
Bieberich believes the lipid that has such a conflicting role in the brain — and that has definitely captured his — will one day be part of a routine blood test and possibly part of treatment as well that, like our cholesterol levels today, gives an early heads-up that something is amiss.
About Dr. Erhard Bieberich
An Impassioned Neuroscientist Even as a teen in Cologne, Germany, Dr. Erhard Bieberich was fascinated by the brain, although his first allure was a bit more esoteric than the hard science that now drives him. Before he ever tried to figure out how to preserve our thoughts by taking on Alzheimer’s, he thought it was just cool how thoughts come to our mind. Most of his mother’s thoughts were of others in this community where he spent his first 35 years. He reasons Hedwig Bieberich knew the names of thousands of people, and he still has vivid memories of them standing in a mile-long line to pay their respects when she died at age 57 after a valiant, long fight with a brain tumor. He remembers as well her tenacity in learning how to walk and to speak again after each surgery. His father, Franz Bieberich, is now in his late 80s, and the son was headed home that next week to see him. The visit was in part because he wanted to help his stepmom ensure that this once super-energetic man — a natural athlete, nonsmoker and healthy eater — was still getting out and about. The short-term memory of his social, previously active father has been slipping for a handful of years, and he is now uncharacteristically too still. When asked if it’s patients that drive Hedwig and Franz’s son, a brilliant, passionate, sometimes impatient and sarcastic individual, he characteristically says that while he certainly wants to help people, it is the science that really motivates him. He laughs and asks why everything in the United States has to have such a human perspective. He then exhales deeply when asked if just maybe his parents’ fight is now his own.