Aggressive, deadly tumors like glioblastoma and metastatic breast cancer have in common a siren call that beckons the bone marrow to send along whatever they need to survive and thrive.
Blocking production of the chemical that initiates that call could be a logical and effective new treatment target, Medical College of Georgia scientists say.
“Our idea is that the most aggressive tumors have the same basic mechanisms of growth and spread,” says Dr. Ali S. Arbab, leader of the Tumor Angiogenesis Initiative at the Georgia Cancer Center and professor in the MCG Department of Biochemistry and Molecular Biology.
They have evidence that an inhibitor of the chemical, 20-HETE, reduces the growth and spread of human glioblastomas and breast cancer in laboratory models.
20-HETE, or 20-Hydroxyeicosatetraenoic acid, is an important part of a healthy body. It’s a metabolite of arachidonic acid, a fatty acid we make and constantly use for a wide variety of functions like helping make lipids for our cell membranes. Its normal functions include helping regulate blood pressure and blood flow. It’s also a known mediator of inflammation, which under healthy conditions can help us fight infection and actually protect us from cancer and other invaders.
But as cancers are wont to do with our body systems, tumors enable too much 20-HETE and the chemical turns on us, first by activating immune cells that will send the cytokines that become the siren call to our bone marrow, says Dr. B.R. Achyut, cancer biologist and a longtime member of Arbab’s scientific team.
“There is normal function and there is tumor-associated function,” says Achyut. “Tumors highjack our system and use that molecule against us.”
High levels of 20-HETE help turn bone marrow cells’ usually lifesaving functions, like making blood vessels as we develop and populating our immune system, against us, Achyut says. Instead, 20-HETE helps bolster the primary tumor site and in the case of breast cancer, for example, helps prepare remote sites – called the tumor microenvironment – for its spread.
Unlike a short-lived call for help from an injury site, for example, cancer’s demand for bone marrow’s support never really ends, says MCG molecular biologist and team member Dr. Thaiz F. Borin.
In this altered state, Arbab and his team have shown that 20-HETE aids activation of things like protein kinases that can change the function of proteins, their location and what cells they associate with, as well as growth factors that can make cells grow in size, proliferate and differentiate. It can even help recruit cells that make blood vessels that enable additional growth. 20-HETE also activates signaling kinases that enable cell division. It encourages inflammation-promoting factors like tumor necrosis factor alpha and several of the interleukins, another class of proteins that help regulate the immune response. In this state, the factors are turning up inflammation to support rather than fight cancer.

Growth is the cue that enables growth
It’s the rapid growth of these tumors that seems to garner such support. Hypoxia, a lack of adequate blood and oxygen often associated with disease and death, is instead a survival tactic for tumors.
When a glioblastoma reaches the diameter of just .1 inch, for example, the spiraling growth and cell division makes the center of this very vascular tumor hypoxic. That leads to the need to recruit from the bone marrow a myriad of factors like vascular endothelial growth factor, or VEGF, that enable blood vessel and tumor growth, which the misguided 20-HETE enables.
The bone marrow cells the tumor cytokines woo also include endothelial progenitor cells to make the lining for all the new blood vessels that VEGF and myeloid cells are making. Myeloid cells are immature, multitasking cells that can drive protective inflammation that, in this case, help tumors suppress the usual immune response in addition to making blood vessels. Increased numbers of these myeloid-derived suppressor cells, or MDSCs, are recruited to both the main tumor and to its metastatic sites. Higher rates of these cells are known to be associated with higher rates of recurrence and metastasis.
“We have to disconnect the tumor from the bone marrow,” Arbab says.
Enter the 20-HETE inhibitor, HET0016, which was developed to learn more about what 20-HETE does. The MCG scientists are using it in their animal models as they suspect it will one day be used in humans, as an adjunct to other cancer treatments.
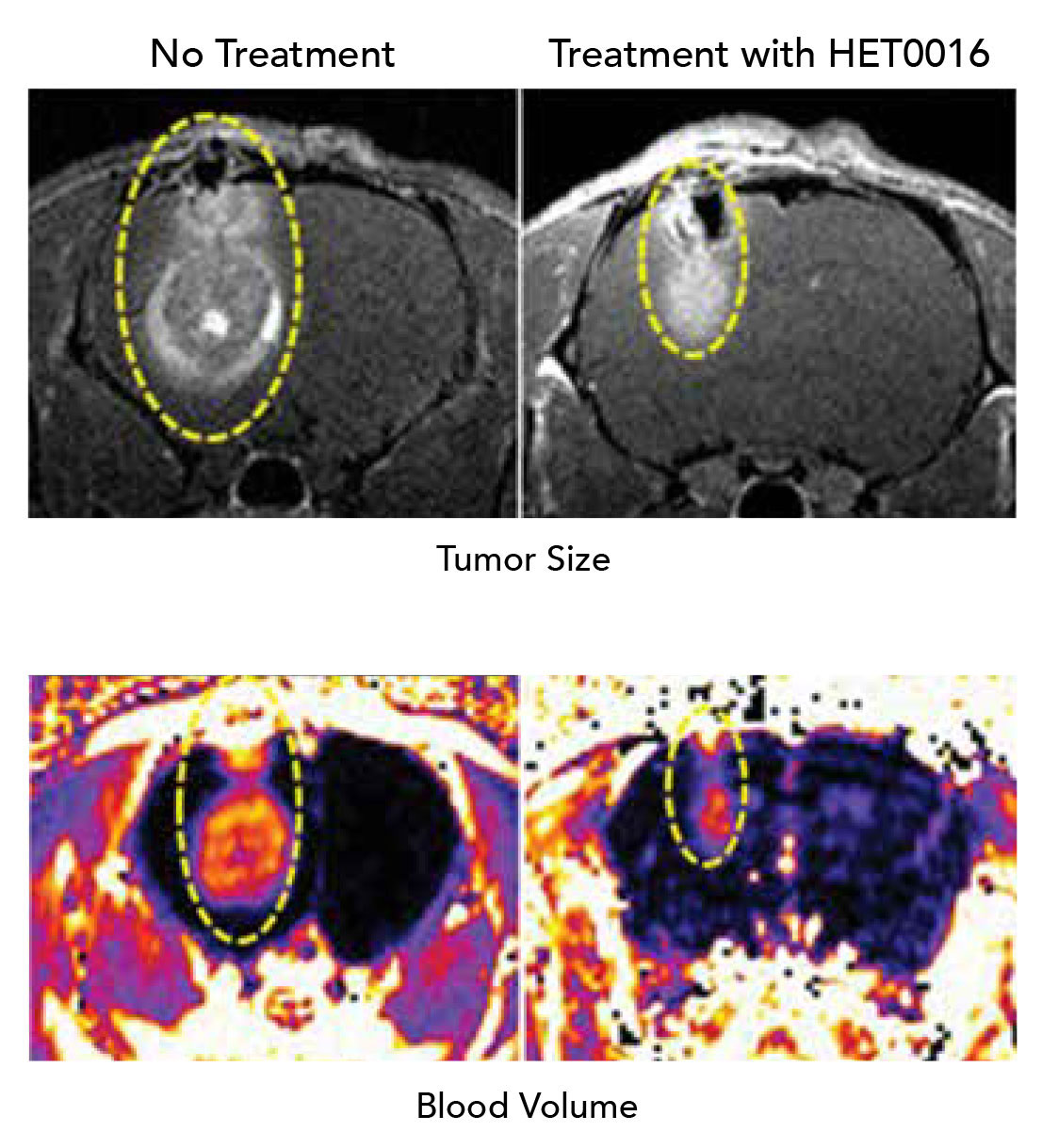
For one study, they gave the drug alternately with the chemotherapy drug temozolomide for three to six weeks. They found in rats with glioblastoma, for example, the rodents survived for at least six months with the tumor instead of the expected few weeks. The scientists don’t know how much longer the rats might have lived because they were euthanized at six months as part of the study. Patients with a glioblastoma have a median survival of about 14.6 months, according to the American Brain Tumor Association.
When they put human breast cancer cells and mouse mammary tumor cells in the mammary fat pad of mice, waited for the cancer to take hold and begin to spread, then intravenously gave mice HET0016 five days per week for three weeks, they found HET0016 reduced the migration and invasion of tumor cells. Forty-eight hours after the drug was given, cancer cells were less able to move about in small test tubes. The drug also reduced levels of metalloproteinases in the lungs, enzymes that can destroy existing protein structures, so that, in this case, cancer cells can penetrate the area and new blood vessels can grow. It also reduced levels of other key inhabitants of the tumor microenvironment like growth factors as well as cancer protective MDSCs. “It gets rid of one of the natural protections tumors use, and tumor growth in the lung goes down,” Arbab notes.
There was less communication between the tumor’s base camp and potential deadly satellite locations in areas like the lungs, liver, brain or bones. When they examined the lungs, they saw fewer cytokines to summon future bone marrow cells and fewer enzymes that also support invasiveness of the breast cancer cells, says Borin.
20-HETE inhibition quietens the cues
“The drug is reducing the ability of cancer cells to create a distant microenvironment where they can thrive,” says Arbab.
In what’s known as the seed and soil hypothesis, Arbab notes that cancer cells are constantly doing test runs, sending cells out into the bloodstream to see if they will take hold at some remote site. About 30 percent of patients with breast cancer, for example, experience metastasis. Glioblastoma, on the other hand, typically does not spread to other organs rather spreads out, increasingly occupying more and more space that should be occupied by the healthy brain. Bone marrow cells’ unwitting contribution to these tumors include growing more blood vessels on their periphery to enable their growing girth. Patients can die from the pressure the tumors put on the brain.
Arbab notes that with both cancers, the primary tumors also shrank with 20-HETE inhibition because they are no longer recruiting the factors that enable growth.
“They are becoming static,” he adds, noting that the 20-HETE blocker does not kill existing tumor cells, rather essentially puts them on hold.
The Georgia Cancer Center team notes that while chemotherapy does kill tumor cells, when the tumors start dying they actually increase their release of cytokines as yet another cry for survival and another good reason to directly target the siren call that tumors are sending, Arbab says.
“Cytokines are the point of action and cancer releases a lot of them,” Borin says of these molecules well known for their role in regulating the immune response.
These days find the scientists working to learn more about how the inhibitor works, including its impact on the tumor microenvironment, and more about what happens to the bone marrow cells that tumors recruit.
They also are looking at exosomes, traveling packages all cells send out as a way to communicate and swap substances. In the case of cancer cells, exosomes appear to be packed with items needed to build the supportive environment for their new distant location in the lungs or elsewhere. Once exosomes establish a niche, they send back a signal to the primary site for cancer cells to join them. The scientists want to further pursue the ability of HET0016 to block these cancer-derived packages.
While a 20-HETE inhibitor has not yet been used in humans, commonly used drugs like aspirin and Celebrex, which target other arachidonic acid pathways, are widely used. The scientists’ studies indicate that healthy bone marrow production is untouched by 20-HETE inhibition because it’s blocking the tumor’s signals to the bone marrow, not the bone marrow directly. And, nobody wants bone marrow cells once tumors get their attention, they note.
“They are working for the tumor,” Borin says.
Notables
Arbab was senior author on separate review articles on metastatic breast cancer and glioblastoma in the International Journal of Molecular Sciences published in December. He and his team published a paper looking at the impact of 20-HETE inhibition on breast cancer in PLOS ONE last summer. The group is responsible for the majority of the published work on the role of 20-HETE in cancer. Research support includes funding from the American Cancer Society and the National Institutes of Health.
The 20-HETE inhibitor used for these preclinical studies is made by Dr. Iryna Lebedeva, assistant professor in the Department of Chemistry and Physics at Augusta University. The MCG scientists already have identified companies that could make a clinical grade inhibitor. To date they have studied only one dose of the research drug, and Arbab has submitted a grant to the National Cancer Institute to look at escalating doses in the rat model of glioblastoma.

Dr. Ali Syed Arbab
Dr. Ali Syed Arbab, 54, was born in Bangladesh, earned his medical degree there at Dhaka University’s Institute of Postgraduate Medicine and Research, then moved to Japan when he received a scholarship to study radiological sciences. Arbab ultimately earned a PhD in that field from Yamanashi Medical University in 1998 and three years later began a postdoctoral fellowship in stem cell and cell tracking at the National Institutes of Health. Afterward, he joined the radiology faculty of Henry Ford Health System and Wayne State University School of Medicine in Detroit. Arbab came to MCG in 2013 to lead the Tumor Angiogenesis Initiative at the Georgia Cancer Center.
His early interests and expertise as a physician and a scientist led him to help develop techniques for keeping up with cells, like cancer cells, as they move about.
He notes that “angiogenesis” in the name of the initiative he now leads is a bit of a misnomer. Because tumors – like glioblastoma and metastatic breast cancer – first just hook up to the tributaries of nearby blood vessels.
But when they grow to a significant size – usually 2-3 millimeters, or .079-.118 inches – and need their own blood vessels, they look all the way to the bone marrow for basic building blocks like VEGF, that help establish our normal vascular network during development. Healthy angiogenesis usually entails recruiting the essentials from nearby, existing blood vessels.
Arbab has documented how stem cells arrive from the bone marrow at the tumor within hours of the first call. Peak accumulation occurs within 24 hours.
But like a badly built house, the blood vessels that tumors rapidly grow are leaky, which would be inefficient for our organs yet are oddly effective for tumors. The leakiness means the tumors always are a bit hypoxic, which serves as that continuous cue to recruit from the bone marrow a variety of factors that ultimately enable tumor growth. The leakiness also means that drugs, like chemotherapy, are less effective at reaching the tumor.
While the majority of work on 20-HETE has been exploring its normal activity, like helping regulate blood pressure, when Arbab and his colleagues first blocked the chemical in the brain tumor glioma, they were surprised to see tumors shrink in response and those leaky blood vessels normalize.
It turns out that the tumor cells actually have less 20-HETE than normal cells, rather it’s the inflammatory cells tumors accumulate releasing the excessive 20-HETE and sending that siren call back to the bone marrow. Newly arriving cells also express a lot of 20-HETE. The tumor microenvironment – the place where the tumor intends to spread – also is seething with it.
He hopes that the increasing evidence of how tumors are manipulating the cells of the bone marrow to grow and spread, will prompt other scientists and clinicians alike to also focus on blocking the deadly manipulation.
“We turn to our bone marrow every day to make things we need to survive. Tumors do the same,” says Arbab, who is working to cut the tie so the tumor can’t thrive, but the patient can survive.





