Test provides unprecedented insight, targets in cancer
There are those three words that most use to articulate love. For these two, there is also “Fight Like Mike.”
“Well, if you’ve ever been told you have cancer, there’s a cold chill that just hits,” says Mike Thames, 35.
It was a Friday in March 2016. Lindsey had just gotten home with their three children. She was still in their front yard in Atlanta, gathering up kids and her thoughts for the weekend.
“I will never forget the moment of him calling me and how it, your life, in one second just changed,” she says.
The clues
Mike was incredulous, but she instinctively knew something big was wrong.
He was putting away Christmas 2015 decorations when the back pain really started. He reasoned maybe he’d pulled something or it was the result of his uncharacteristic sedentary state over the holidays. The pain came and went. He got anti-inflammatories and muscle relaxers on the first doctor visit. But the pain mostly persisted. The day he went back to the doctor months later, he couldn’t really walk because the back spasms were so bad. “I thought I had ruptured discs or something,” he remembers.
An MRI requested by the spine surgeon first raised the specter of cancer. Mike thought, “No way,” but he knew he had to call Lindsey. Then nothing really showed up in the bloodwork done by an oncologist he managed to see still later that afternoon.
But a battery of tests a week later at Emory University confirmed a cancer.
Mike had never heard of this mixed lineage leukemia, a rare and aggressive presentation of acute leukemia. It’s called mixed because it is a combination of the more common and treatable acute myeloid leukemia and acute lymphoblastic leukemia.
At Emory’s Winship Cancer Institute, Mike would also find Dr. Vamsi K. Kota, a hematologist/oncologist, who draws a “whew” from his patient as the “man who saved my life.”
Kota calmly told him that he hoped that he didn’t have any weekend plans, because they needed to get started. The young man who had only ever been in the hospital to be born and that one other day when he broke a bone, would spend about 90 days in the hospital that year.
The beginning
Mike is the youngest of three who grew up in tiny Reserve, Louisiana, and who met Lindsey while they were both at Auburn University. He moved to her hometown of Atlanta in 2008 and they married a year later.
“There was something about him,” Lindsey says. “He has a strong will and a perseverance and a fighting spirit that I fell in love with from the start. He knew what he wanted in life,” but he was also kind and fun in the process, she says. They knew pretty quickly that one thing they wanted was to be together.
Mike had grown up with his family’s NAPA Auto Parts store, so he had a lifetime love of cars and tinkering with engines. Lindsey’s family owned and operated a chain of new car dealerships. Mike would become general manager of the Pope family’s Chrysler, Dodge, Jeep and Ram dealership in Stone Mountain, Georgia. He loves the high-energy human contact and long hours in this super-competitive business where he is boss to north of 80 employees. “All these people feed their families based on our success here,” he says, then pauses the conversation for a minute to talk with a longtime customer who had brought him breakfast.
They would have professional success and fun and three children, their youngest and the wildest of the bunch, toddler Graham “Tornado” Thames; first born, Jake, who turned 8 in October, three days after his father’s birthday; and “Princess” Celia, now five.
The “Tornado” was just six months old when cancer also moved into their lives.
Lindsey saw Mike’s fighting spirit rekindled that day, and together, with family and friends, they would begin to Fight Like Mike.
The options
The mixed lineage of the leukemia inside him made it hard to find a good target and made the cancer tough to treat. Kota used 10 different drugs, including chemotherapy and immunotherapy, but they didn’t really touch it. A Nov. 7 bone marrow transplant, thanks to the largesse of a 21-year-old donor Mike lovingly calls the “other Mike,” worked pretty well for about 10 months. Mike went into the hospital on Halloween, got home after Thanksgiving, but made it back to work in 100 days.
By August 3, 2017, Mike was feeling like maybe he had pneumonia, but it was the cancer.
The young couple knew that a year of transplant success was an important hurdle and that they were short. “Mike couldn’t walk at that point. I wanted to crawl in a hole,” says Lindsey.
“My man went back into action, he went back into action quick, too,” Mike says of his doctor Kota.
“When the disease comes back after transplant, it’s never good,” Kota says. He remembers the day when the dynamic young man came in hunched over in a wheelchair. Kota knew, even before the scan.
This blood disease often inexplicably comes back as what Kota describes as a ball, a mass of leukemia cells called myeloid sarcoma. Mike now had a couple of masses in his pelvis, both in and near bone. When either leukemia or sarcoma show up following transplant, the one-year survival is about 25 percent.
This time Mike also had graft versus host disease, a double-edged sword because it proves the transplanted immune system from the other Mike is strong, but part of what it is now attacking is patient Mike’s own tissue. Symptoms can include gastrointestinal and liver function disruption and more. Mike’s problems were with his lungs and a significant, painful rash. Still, the aggressiveness of his new immune system was also helping him battle leukemia. It’s a common conundrum for bone marrow transplant patients and their doctors.
Either the leukemia or graft versus host disease alone could be lethal. “We needed a plan for controlling both of them,” Kota says, something that would both help protect his lungs and destroy the leukemia.
Patients with graft versus host disease often end up on immuno-suppressive drugs, which suppress their new, aggressive immune response, but that means the leukemia could have an easier time of it. And Mike’s disease, mixed lineage leukemia, was particularly aggressive.
Another transplant was not the answer, because the new immune system was clearly working. Kota had other options for Mike in his head, but he wanted more. He thought about his friend and colleague Dr. Ravindra Kolhe. The two had trained about the same time at the Medical College of Georgia and AU Health, Kota in hematology/oncology and Kolhe in pathology. They met by chance on an elevator one day. They both love science and hate cancer, and would become fast friends.
Kota first blasted the masses with radiation, but experience taught him that they essentially always come back. So he also talked with Mike and Lindsey about sending a new biopsy to Kolhe, now director of the Georgia Esoteric and Molecular Laboratory in the MCG Department of Pathology, so they could examine the genetic makeup of the recurrent disease.
Mike’s faith was unwavering. Doctor and patient had taken to each other long before then.
“He had me at hello, how about that,” says Mike. “I think we just fit well together. He’s got small kids, a beautiful family, he just knew what we had.” So Mike customarily told Kota, “Whatever you need.”

The fight
Kolhe is a molecular pathologist, who, before he reached age 40, was tired of giving patients and their physicians incomplete information about a cancer diagnosis. He wanted to give them more than a cancer type and its stage. He wanted to give them more of a bull’s-eye. His field was already moving from looking for the most suspect gene in a cancer type to help make the diagnosis and determine treatment, to looking at several and sometimes many suspect genes. Evidence showed that causative gene variants may not be the same even in two individuals with the same cancer type, like melanoma or lung cancer. Conversely they may be similar in patients with different cancer types.
Kolhe wanted to look as broadly yet specifically as he could. He had worked with San Diego-based biotech company Illumina, Inc., to develop the TruSight Tumor 170 panel, the most comprehensive genetic analysis out there at this writing. It simultaneously assesses six known cancer-causing variants in the DNA and RNA of 170 genes associated with 14 cancers, including breast, ovarian, skin, bladder as well as pancreatic and liver cancer, which are both on the uptick in this country.
This next-generation sequencing, or molecular profiling of tumors, also looks at changes in a handful of genes important to the normal – and continuous – DNA repair, or homologous recombination. These changes can leave the genes at increased risk for even more mutations and reliant on less-precise repair options that increase the risk that a normal cell becomes cancer. The more aggressive and obnoxious the cancer, the more variations, is the way it tends to go, Kolhe says.
The comprehensive test generates huge volumes of information on a single patient. So he also worked with bioinformatics giant IBM Watson Health to come up with a way to analyze the large amount of data and identify what all is abnormal and now likely oncogenic, meaning genes whose machinery has changed so that the normal life and death cycle of cells just doesn’t happen.
IBM Watson for Genomics then looks around the world for drugs, both those already approved and those still under study, which target the abnormalities. The icon of artificial intelligence also groups the gene variants by pathway with the idea that multiple genes that use a similar pathway should be a major player and sound targets for the cancer. That pathway perspective is one of the best parts of the test from Kota’s perspective, because it provides, not definitive information, but definitely strong clues about the most efficacious strategies.
Kolhe says it might take 10 people 10 days to do this type of thorough assessment for a single patient. In fact, as part of the extensive validation process for the entire system, many people did this sort of exhaustive search behind Watson and found him true essentially 100 percent of the time.
The new test, Augusta OncoTarget, provides the comprehensive assessment in about 20 minutes. Mike was the second patient evaluated for treatment.
Genomic sequencing and analysis hold great promise in extending life expectancy and improving quality of life, says Stephen Harvey, vice president for oncology and genomics at IBM Watson Health. But to date, access to precision oncology services has been relatively limited. This is due in part to the data overload that physicians face in delivering genome sequencing and analysis, Harvey says. As examples, there were 28,000 medical journal articles pertaining to cancer in 2007 and more than 120,000 in 2017, he notes.
So IBM Watson for Genomics essentially does the data analysis for them, analyzing genome sequencing data, and comparing that data against these massive bodies of genomic, clinical, scientific and pharmacological databases to help uncover potential therapeutic options that target the tumor’s genetic alterations, Harvey says. Then, Watson produces a report for physicians that identifies genetic alterations that are actionable based on the literature as well as drugs and clinical trials that target those alterations.
“Making precision medicine a reality for patients like Mike is the epitome of our mission at IBM Watson Health,” Harvey says.
The Watson
The couple look up from their smartphones and at each other, grinning at the tension relief provided by talking about Watson rather than leukemia. When they first heard the name Watson, they thought he was another person Kota wanted them to see. While they laugh, there is a more gut-wrenching relief provided as well.
“It’s indescribable … feeling like there’s another chance. We have something else,” says Lindsey. “It’s not the end of the road.”
“All these years we have treated different tumors differently, like brain or breast cancer. Treatments were not necessarily based on causative genes, rather whatever we could find in the lab that would stop the cells from dividing,” Kolhe says. “That’s one of the reasons some patients respond to a therapy and others don’t. Something else was driving their tumors.”
Mike’s 41-page study revealed a myriad of gene changes known to contribute to cancer and another handful whose cancer role is still unclear. The succinct summary provided two clear targets active in his disease.
Variations in four of Mike’s genes activated the PARP pathway, which enabled the cancer cells to repair any DNA damage that might be lethal to them, including damage done by treatment. Variations in two other genes activated the mTOR pathway, a regulator of metabolism and physiology that helps cells survive and divide, which is also critical to cancer cells.
IBM Watson for Genomics suggested – and Kota agreed – that drugs to inhibit both would be bad for the disease and good for Mike.
Kota settled on a combination of three drugs, two of which ultimately target both the leukemia and the graft versus host disease. The third primarily targets the leukemia.
It’s a unique cocktail for Mike that had not been mixed before. But like Kota’s patient, like every patient, Mike and his cancer were distinctive.
“He’s told us all along that you can’t treat the name of the disease, you have to treat the patient,” says Mike, so it made good sense to this advocate of employees and customers alike that his doctor wanted to target his specific problem.
“We are giving them a window to look at what is driving the relapse, resistance and metastasis,” Kolhe says. “We want to provide a window for every patient we see, whether new, relapsed or chemotherapy resistant.”
Kolhe gives the example of a 36-year-old woman with meta-static breast cancer that is estrogen-receptor positive but had not responded to the often-effective hormone therapy. Augusta OncoTarget detected eight genes affected in different ways, including a mutation in her estrogen receptor that made her unresponsive to what would be considered a solid treatment choice.
Kolhe called her physician, recommended that he stop hormone therapy and gave him other targets.
Still bigger picture, Kolhe and Kota hope the clues they are gathering in these early years will not only help individual patients like Mike now, but will contribute to the knowledge about what gene variations are the biggest factors in cancer and need to be the focus of drug development, if drugs don’t already exist. It also should help identify new problematic gene variants.
They note that most problematic variants don’t appear to be passed along from one generation to the next. Rather, most result from what we come across in life, including cigarettes and alcohol we consume, and environmental toxins that we often don’t even recognize, even how and how often sunrays hit our face.

The fight of Mike
In July 2018, Kota returned to the faculty of MCG and the Georgia Cancer Center as director of the Bone Marrow Transplant Program. Mike and Lindsey came with him.
In August 2018, one year out from the start of his innovative therapy, the PET scan again showed Mike was cancer free. Kota plans to continue treatment for one more year, calmly acknowledging again both this leukemia’s aggressiveness and that passing time is a good thing. Like his patient, Kota is optimistic, even in this formidable battle.
Kota visited Portugal for the first time right after Mike was diagnosed and walked a trail along a mountainside that frankly looked like heaven to him. September 2019, two years after relapse, Kota has a date to walk it with Mike.
While Mike googled his doctors to check them out, he told them early on that he didn’t want to know more than was necessary about what was happening to him. Yet, he can recite what has happened. In fact, although he is functioning, typically wide open, his mind never strays too far from cancer and what is next in his fight. While he wishes he didn’t have to think about it, he doesn’t really tire of it because he is ultimately thinking about his family and his life.
“Some days, I want to cuss at it,” says Lindsey. “I am so mad at it for … the pain and the heartache — and believe me, it is a heartache every day.” Other times, she wants to forgive cancer and is oddly grateful for the new life perspective it has given her and Mike.
“I love him now more than I ever thought I would,” she says. She thinks about that pivotal day in their front yard when she more fully embraced that love, the possibility of its loss and the courage of the man who stands beside her.
“It’s been awful, awful, and yet you keep going because of the hope in this,” Lindsey says. “I think we live for hope.”
And, for another clear scan that results from fighting like Mike.
Find out what it’s like to “Fight Like Mike” at fightlikemike.org.
1,000 Mikes
When Kolhe presented Augusta OncoTarget at the Association for Molecular Pathology meeting last year, he sensed a level of astonishment that this type of detailed analysis could be done outside big, typically commercial reference labs.
He also found a clear desire for it. Kolhe is now leading the 15-member international group, Sequencing and Oncology Informatics Academic Team, or Sequoia Team, which includes Moffitt Cancer Center, the University of Michigan and other institutions who want to offer the test to their patients and others. Those facilities will use the 170-gene panel packaged with Watson or other bioinformatics systems for analysis.
He and Kota are working with Illumina again as well as other biotech companies to rapidly expand the scope of the test to about 500 genes.
Their many goals include making molecular bull’s-eyes available to patients and physicians at the initial diagnosis. Because of cancer’s ability to change in response to treatment, the analysis could also one day be used periodically to help detect if the bull’s-eye has moved.
So will 1,000 Mikes. The developing philanthropic initiative of Kolhe, Kota and now Lindsey and Mike will enable 1,000 individuals from across the globe, who might not otherwise have access, to receive the $3,000 test.
“That’s all we could ever dream of for the rest of our lives, is to give to something that would help other patients,” Lindsey says, so that each patient knows, ideally at the starting gate, the likely best treatments against the cancer they battle.
In October, Kolhe was notified of GEM Lab’s selection as a CLIA-certified/accredited laboratory for the NCI-Molecular Analysis for Therapy Choice (NCI-MATCH or EAY131) precision medicine trial. The trial is being co-led by the National Cancer Institute, part of the National Institutes of Health, and the ECOG-ACRIN Cancer Research Group (ECOG-ACRIN).
Under the terms of the collaboration, MCG will reach out and notify treating oncologists from the more than 1,100 clinical sites participating in the trial when the lab identifies gene abnormalities that could make a patient eligible for one of several NCI-MATCH treatments. Tumor gene testing by a designated lab is the only pathway for patients to enroll in the trial.
To learn more about NCI-MATCH, including clinical trial sites across the country, visit
ecog-acrin.org/nci-match-eay131.
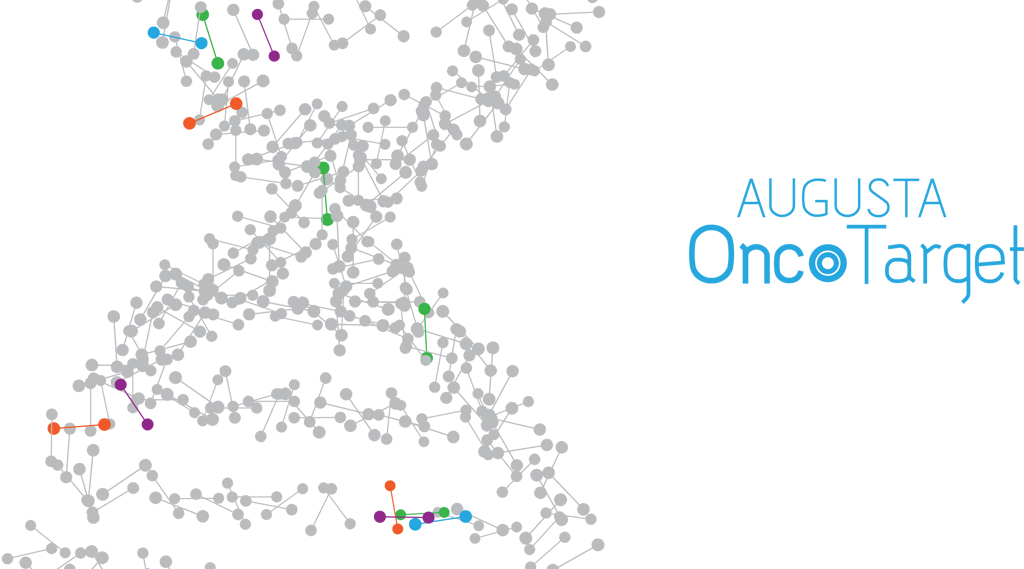
Augusta OncoTarget, one of the latest additions to MCG’s Georgia Esoteric and Molecular Laboratory, includes next-generation sequencing that enables large numbers of genes, and an unprecedented number of known cancer-causing variants in those genes, to be tested simultaneously.
IBM Watson for Genomics then performs a genomic interpretation of the large amount of data generated to help identify the patient’s variants as well as drugs, which are already in use or in clinical trials, known to target them.
Augusta OncoTarget includes the TruSight Tumor 170 panel that simultaneously looks for six different variant classes in both the DNA and RNA of 170 genes in a patient’s biopsy.
Within a few minutes, IBM Watson for Genomics identifies gene variants relevant to cancer in the patient sample and associates drugs that target the abnormalities based on the medical literature.
The 170 genes in the panel are commonly altered in a wide variety of cancers in adults and children including brain, breast, lung, bladder, prostate, colorectal, pancreatic, liver, gastric, thyroid, skin, ovarian/uterine, soft tissue cancer like rhabdomyosarcoma as well as leukemia.
Variants detected in the DNA include single nucleotide variations that enable the uncontrolled cell proliferation that is a cancer hallmark. They also include the number of copies of a gene, known as amplifications, since multiple copies can dramatically increase the amount of protein produced and aid cancer.
Augusta OncoTarget also detects splicing variants, an alteration in the DNA sequence – the normal order of the letters A, C, T, G – which can alter the proteins produced and the function of genes and cells, enabling, as an example, a healthy cell to become a cancer cell. It also finds insertions and deletions in the DNA sequence, a common genetic variation, which can alter gene function and contribute to disease.
Variants detected in the RNA include gene fusions, since genes normally do not fuse, and their joining can result in the activation of typically silent genes and cancer.
“We look at six different categories of variants in both the DNA and RNA of each gene because cancer can result from all of them,” says Dr. Ravindra Kolhe, molecular pathologist, director of the GEM Laboratory in the MCG Department of Pathology and primary developer of the new test.
“We believe this comprehensive analysis will help provide scientifically sound and personalized therapy targets for consideration by patients and their physicians.”
Kolhe worked with California-based biotech company Illumina, Inc., and IBM Watson Health, headquartered in Cambridge, Massachusetts, to develop Augusta OncoTarget.