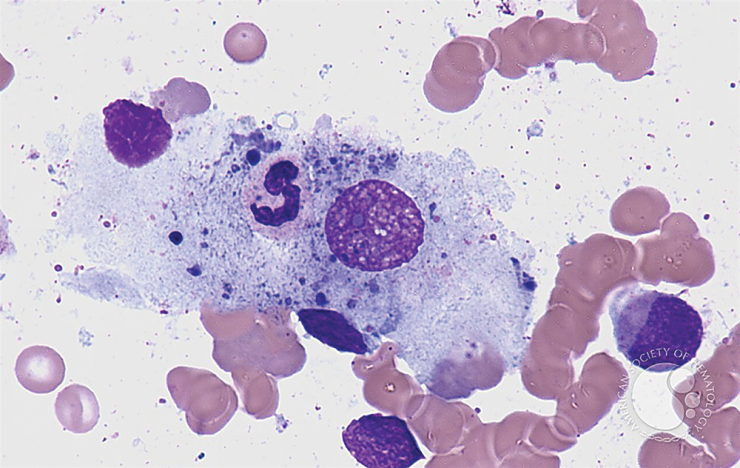
Like our brown eyes and the angle of our chin, our innate immunity is something we are born with.
It’s the frontline and fundamental ability of our bodies to quickly and consistently attack a foreign invader, like SARS-CoV-2, or a bacterium that creeps into a cut on our finger.
Our adaptive immune system is more personal and targeted, a kind of learn-as-you-go protection our bodies develop as we live our lives and get exposed to antigens, substances which our bodies don’t recognize as our own, like that COVID-19 virus.
 The troops in each system are different but clearly connected.
The troops in each system are different but clearly connected.
Our skin and eyelashes are part of frontline innate immunity, that also includes immune cells called monocytes, which can become macrophages that can literally surround and consume an invader. There are also neutrophils, which also travel through the blood to the site of an infection, where they too can kill and ingest an interloper.
Adaptive immunity, for the longer fight, includes T cells, B cells and those antibodies our B cells turned plasma cells produce against a specific target. How well the innate immune system determines what is wrong and informs the T cells of the problem determines how well the T cells mount a long- term response.
But like many things in life, these formidable protectors have a tradeoff: They also can cause disease.
“The immune system is involved in protecting your body, but it can also be manipulated by cancer cells or by viruses to work against you,” says Dr. Catherine “Lynn” Hedrick, co-director of the new Immunology Center of Georgia being established at the Medical College of Georgia.
Hedrick hesitates maybe a millisecond then says “no” when asked if there is a disease she can think of in which the immune system is not a player, whether in trying to combat disease or in, unfortunately, sometimes triggering illness. Certainly the immune system plays keys roles in the nation’s most common causes of death, heart disease and cancer, which are the focus of the work of Hedrick and Dr. Klaus Ley, center co-director.
Hedrick and Ley are both vascular biologists and immunologists studying how and why the immune system crosses the line, producing excessive, protracted inflammation, which at lower levels and for shorter periods of time is an essential response to an invader or to help heal a wound. But in excess, inflammation can dramatically increase the development of blood vessel disease throughout the body.
They also are looking at the flipside of how to help the immune system better perform its protective roles, like consuming a melanocyte, a cell which normally gives our skin its hue, that starts to reproduce wildly into melanoma cells.
roles, like consuming a melanocyte, a cell which normally gives our skin its hue, that starts to reproduce wildly into melanoma cells.
Recruited to MCG from California’s famed La Jolla Institute for Immunology, the two are leading an initiative that is already attracting other new talent and pulling together long-time stars like Dr. David Munn, 1984 MCG graduate and co-director of the Pediatric Immunotherapy Program at the Georgia Cancer Center and Children’s Hospital of Georgia. The pediatric hematologist-oncologist’s basic science helped uncover a novel enzyme used by tumors to protect themselves, called indoleamine 2,3-dioxygenase, or IDO, and an IDO inhibitor is now in studies to battle intractable childhood brain cancer. Like, Dr. David Fulton, director of the Vascular Biology Center, which has a focus on many of the same atherosclerotic killers like heart attack, heart failure and hypertension. The federally funded vascular biologist has led the center since 2013 — he served as interim two years before that — and his studies include exploring causes and better solutions for problems like the dangerous thickening of blood vessel walls that occurs in pulmonary hypertension and resulting heart failure.
“It’s a once in a lifetime opportunity both from the resource side and from the environment,” Ley says of the move to Augusta. “I see a lot of good things happening here. (Augusta University) President Keel is excited about it. Susan Shows is excited about it. I really look forward to helping bring immunology into many aspects of science here, vascular, neuro, autoimmune, and cancer of course,” says Ley. “Really linking these various disciplines is fantastic,” says Hedrick.
Shows is president of the Georgia Research Alliance, which supports research and entrepreneurship at research universities in Georgia, including helping recruit stars like Hedrick and Ley, who have both been named GRA Eminent Scholars.
The pair’s goals for the new center include not just better understanding how the immune system works in sickness and in health, but also helping improve therapies and diagnostics, and educating the next generation of immunologists.
First on the scene
Hedrick’s primary target is monocytes, “cool” cells that do “nothing but recognize invading things, things that are not supposed to be there,” she says of these frontline immune responders. She zeroed in on monocytes while studying blood vessels and the endothelial cells that line them. Monocytes as well as neutrophils, another white blood cell she studies, can bind to endothelial cells early in infection and disease and she liked that they were first responders directing the scene in the attack against a virus or a cancer cell.
But as she, Ley and others are learning, the cells also work together in diseases like atherosclerosis. Her lab has identified unique populations of monocytes, moving the bar from traditional thinking that there were basically two types of monocytes — one that was inflammatory and another nobody really understood — to recognizing that the healthy among us likely have more like eight different monocyte types. Those with cancer or atherosclerosis appear to have even more. Just what they are doing she is still working to understand, but she thinks these monocytes may be influenced by the microenvironment of the tumor or plaque, maybe in a good way to help but potentially to also be deleterious.
“I think that is where the field is. We have these great cells, we know the really critical functions of some of them, but we also have these new ones that we don’t understand yet,” Hedrick says.
Her work has shown that in patients with cancer, heart disease and COVID-19, the monocyte complement definitely includes unique populations that are particularly good at an attack. Some of the most specialized of these “nonclassical monocytes” are those engineered to kill viruses or tumors cells. These monocytes are always circulating in the blood, hunting for these troublemakers.
She has shown, for example, in collaboration with Dr. Richard Hanna, formerly at La Jolla and now associate director of bioscience immunology at AstraZeneca, that in lung cancer, some of these wandering cells move in, and help recruit other immune cells that can kill lung cancer cells and block metastasis while monocytes scarf up tumor cell debris, in work published in the journal Science.
The other side of the monocyte coin is taking those cells that are attacking cancer and helping them attack better and/or faster.
She is working with colleagues at the National Institutes of Health Clinical Center in Bethesda, the largest hospital devoted to clinical research, to start from scratch and engineer monocytes that are more proinflammatory and hence more likely to kill cancer.
Hedrick also is working with colleagues at the National Cancer Institute on cell-based therapies that instead reengineer monocytes for better killing, much like CAR T-cell therapy, already used to treat blood cancers, that includes removing a patient’s T cells, modifying them to better attack their cancer, then returning them.
With physician scientist Dr. Rosandra N. Kaplan at the NCI Center for Cancer Research, she is exploring the mystery of why today CAR T-cell therapy works so well for some patients and fails others and if monocytes are part of the equation.
“We have found with Dr. Kaplan that in this population there are different monocyte profiles in patients where the CAR T cells expanded and worked well versus the ones where they didn’t expand and didn’t work. It’s night and day,” Hedrick says.
“If we can learn how to either make more of them or make them work better, meaning seize the tumor faster or orchestrate it killing better, that is a good thing,” Hedrick says.
Hedrick also brings with her a new $13 million NIH Program Project grant renewal that is enabling a deeper dive into just what this unique subset of human monocytes she’s found are doing and how they are doing it in both sickness and health.
Her colleagues on the major, new initiative include Ley, Dr. Coleen A. McNamara, cardiologist and clinician scientist at the University of Virginia whose expertise includes the immune system’s regulation of cardiometabolic disease; and Dr. Yury Miller, an MD/PhD and professor in the Division of Endocrinology and Metabolism at the University of California, San Diego, and an expert on toll-like receptors, which are skilled at recognizing invaders as well and helping link up the innate and adaptive immune response, as well as macrophages and inflammation.
They are looking in the blood of humans with a little atherosclerosis, which represents “normal” and most of us, and those with a lot, which means they likely will end up in a cardiac catheterization lab and potentially in surgery.
One focus is CD9, a member of the tetraspanin family of proteins whose many functions include helping organize receptors in the cell membrane to enable cell signaling. Their location means they can also modulate immune receptor activity, and when the scientists look down their action pathway they find they induce a lot of factors involved in turning up the immune response. While CD9 likely needs this skill set to help cue the elimination of invaders like a virus, they think the machinations also can drive plaque formation. And, they have found that some monocytes have a lot of CD9 while others have very little, Hedrick says, of the surprisingly striking differences.
“…in a tissue, in a microenvironment, in a tumor, in a plaque…all of these different immune cells are constantly talking to each other and we think themonocytes and possibly the neutrophils talk to the T cells very, very early and turn them on or off.”– Dr. Lynn Hedrick
Monocytes are generally skilled at becoming macrophages, but they’ve also found the first evidence that monocytes that have high expression of CD9 produce high-expressing CD9 macrophages, which they have found in plaque. They theorize that these unique monocytes and macrophage popuations can promote atherosclerosis, and part of that includes getting T cells and B cells to come onboard. They also want to know whether CD9 expression is really the distinctive difference.
“We will be digging down deep at what makes CD9 monocytes different from other monocytes and if this is something we could target in a drug or some other way to help monocytes work better,” Hedrick says.
That includes CD9’s impact in cancer. There is some evidence that their resulting proinflammatory state may actually help fight against cancer. “It might have the opposite effect, we don’t know,” she says of different responses to these cells that may play out in the nation’s top two killers. There is clear evidence of the polarity with other immune cells, like those regulatory T cells, which tend to bring down inflammation, which is good in atherosclerosis and bad in cancer.
Bigger picture, the close collaboration of the four labs with experts in each immune cell type all looking at the same group of patients’ cells and disease states will also enable studies to try to understand the crosstalk between the key players — monocytes, macrophages, T cells and B cells — and the innate and adaptive response.
“It’s kind of like a silo of studying cells,” she says of how scientific studies can play out. “But in a tissue, in a microenvironment, in a tumor, in a plaque that is not how it works. All of these different immune cells are constantly talking to each other and we think the monocytes and possibly the neutrophils talk to the T cells very, very early and turn them on or off,” Hedrick says.
When good goes bad
Ley’s focus has been on how our immune system contributes to atherosclerosis, where plaques deposit on internal artery walls blocking the vital passage of blood and the nutrients and oxygen it carries throughout our body. He has looked at how those monocytes that intrigue Hedrick, can adhere to the lining of blood vessels and contribute to disease. At how genetics and diet can make regulatory T cells, which should tamp down inflammation, accelerate it, as well as a host of other twists and turns, including how exposure to a virus or bacteria can contribute to atherosclerosis, in addition to the culprits we know like consuming a lot of saturated fat.
Atherosclerosis is not an autoimmune disease, like type 1 diabetes or lupus, he notes, still you cannot understand it without immunology. “It’s a vascular disease with an obligatory autoimmune component, and the autoimmune component develops later, not when you start the disease, not when you have your first plaque.”
Still, he can tell just from looking at the cells moving about in our blood, those monocytes and T cells, B cells and macrophages, collectively termed peripheral blood mononuclear cells, which are exposed to what we are exposed to, whether we have cardiovascular disease.
“The immune system makes it worse,” Ley says, and his long-held interest in the increasingly clear connection comes from the growing ability to definitively manipulate the immune system. Manipulating the diet, which would seem comparatively simple, is not as simple as you think.
No doubt, other interventions are needed. While drugs like commonly prescribed statins can reduce LDL levels, heart disease keeps its seat as the top killer in the world, he and his colleagues wrote in 2019. Those statins have been Food and Drug Administration approved since 1987.
Ley has sound evidence that a vaccination that takes the immune system out of the equation could be safe and help keep blood’s vital passageways open.
Vaccination is a good approach because it is specific for the problem, in this case a piece of the LDL, or bad cholesterol, without impacting other protective immune responses and while providing long-term protection, Ley and his colleagues Drs. Kouji Kobiyama and Ryosuke Saigusa at the LaJolla Institute , wrote in 2019 in Current Opinion in Immunology.
He notes that LDL is another two-edged sword, providing the free fatty acids we burn so we can literally run for hours. LDL becomes a problem potentially because of our genetic predisposition, our diet, our inactivity and our obesity, and resulting high blood levels of LDL accumulate on our arterial walls, become oxidized and start sending out pro-inflammatory signals.
Apolipoprotein B, or ApoB, the core protein of LDL that helps hold it together, enabling fat and cholesterol, some of which we need, to move through the blood, becomes the target.
Even the healthy among us have T cells that recognize “snippets” of ApoB, he says, noting that for some reason central tolerance, which should rightly eliminate T cells that recognize ApoB, doesn’t completely work. A similar thing happens in, for example, type 1 diabetes, when our central tolerance against insulin is very weak, Ley says.
reason central tolerance, which should rightly eliminate T cells that recognize ApoB, doesn’t completely work. A similar thing happens in, for example, type 1 diabetes, when our central tolerance against insulin is very weak, Ley says.
His lab was the first to identify the ApoB-specific immune cells, CD4+ T cells, known to help coordinate the adaptive immune response, that in the face of high cholesterol are prompted to produce a lot of inflammation-promoting cytokines, small proteins known to have a role in cell signaling, including adjusting the immune response. Their studies of patients at the University of Freiburg, in Germany, ages 43 to 90, who did and did not have coronary artery disease, indicated that those with clinically relevant disease had a lot more of these cells.
Healthy mice had some ApoB-specific T cells, but they also had those inflammation-dampening regulatory T cells, they reported in 2020 in the journal Circulation. His studies also indicate that while newborn mice don’t have these ApoB targeting T cells, a few days later, they do.
As a result, macrophages and the smooth muscle cells, which provide strength and flexibility to blood vessel walls, proliferate, more immune cells move in from the circulating blood and a plaque is born in our heart, our legs and/or our brain.
“The autoimmune response amplifies the response,” Ley says now.
Like with everything in science and medicine, a timeline is hard to pin down, but he says first in human trials in healthy volunteers for the vaccine, the norm for studies of new treatments and vaccines, should be doable in about five years.
Those of us still healthy and likely relatively young with a family history of heart disease should be a good target for this tolerogenic vaccine that should take amplification out of the equation. Even the very young, if the findings of efficacy and safety hold, might benefit so they never develop disease.
Next steps include formulating a vaccine for these individuals as well as mice then seeing whether it works in human cells and the lab animals. Ley is currently in talks with companies in Belgium, Texas and Illinois in collaboration with Atherovax, a biotech company he founded in 2018 with famed cardiologist Dr. Eric Topol, director and founder of the Scripps Research Translational Institute, and Dr. Clive Meanwell, founder and former CEO of The Medicines Company, which developed the LDL-lowering drug Inclisiran (the company was purchased by Novartis in late 2019), who is now CEO and founder of Population Health Partners, a global investment firm focused on health technology.
“I think about it all the time,” Ley says of the vaccine that could help unseat heart disease.
Early days
Growing up in Monroe, North Carolina, Hedrick loved to study science and the solar system. Then her mother, Margaret Hedrick, was diagnosed with type 1 diabetes. Hedrick knew at age 7 that she wanted to be physician or a scientist to help cure her mom, who would experience significant vascular complications, which ultimately led to the loss of her legs and cost the substitute school teacher her life at age 65. Hedrick was 20 when she lost her mother who had always encouraged her to excel. As she worked to decide what to study, she arranged an internship with famed endocrinologist Dr. K. Patrick Ober, an expert in type 1 diabetes, who is still a professor at Wake Forest University School of Medicine, and she realized there that her nature would likely make her a more effective scientist than physician, so she started graduate school there.
It was ultimately the more than 60,000 miles of blood vessels and the immune cells swimming inside those vessels that captured her. The vascular disease that took her mother’s legs led her to cardiovascular disease, a disease in its own right, but also a complication of others like type 1 and 2 diabetes and even cancer treatment. In fact, she would identify an accelerated incidence of cardiovascular disease in type 1 diabetes, which was previously just associated with the more common, often lifestyle-related type 2 diabetes.
Hedrick earned her PhD in biochemistry from what was then Bowman Gray and completed postdoctoral work at the Molecular Biology Institute and Division of Cardiology, at the University of California, Los Angeles. Today, she loves working with her physician colleagues because the collaboration really drives new therapeutics and cures and a focus on the needs of patients that includes the human material that they study.
“If you really are interested in science and helping people, then at some point you realize you need to study human cells and human tissue,” she says, acknowledging the difficulty obtaining those valuable samples. But the human blood that she, Ley and their colleagues now use to study the immune cell complement and differences in sickness and in health, is thankfully more accessible.
“If someone in the community wants to give us a blood sample, we can take that blood sample and learn a lot about the immune cells and how those work in keeping you healthy, but how they also can change when you have cardiovascular disease, or change when you have type 1 diabetes,” she says.
The last two years of high school in Ley’s native Germany are like the first two college years in the United States and he was majoring in mathematics and physics. A teacher told him then he would become a scientist even though Ley said he wanted to be a physician. An old girlfriend told him he might not be a good doctor.
“They knew me better than I knew myself,” Ley says. He realized they were correct when he went to medical school at Julius-Maximilians-Universität, in Würzburg, and started doing research in the Department of Physiology halfway through. He finished medical school because he needed to, he says, then in 1992 he earned a habilitation in physiology at Freie Universität Berlin, a high degree developed in Germany centuries before, that is heavy in research and required to rise to the top ranks in the German university system. He also completed his postdoctoral fellowship at Freie Universität, then came to the United States for a couple of years as a visiting research scientist at the University of California, San Diego Department of Applied Mechanics and Engineering Sciences—Bioengineering before going back to Freie Universität. He came back to the US and the University of Virginia as an associate professor at one of the oldest and best biomedical engineering programs in the world. He became a biomedical engineer by “immersion” and some happenstance, and liked the creative aspect of making something that previously did not exist.
He uses that innovative approach in his science to this day, but his physiology background, studying small blood vessels and muscles, drew him back and in 2001 he was named co-director of the Cardiovascular Research Center at the University of Virginia. He would remain in Charlottesville until moving to La Jolla in 2007 after his work in atherosclerosis made him realize he needed to also become “immersed” in immunology. He would become a pioneer in vascular immunology.
“You cannot understand atherosclerosis without immunology,” he says, acknowledging that not everyone is yet a believer. But with mounting evidence that, for example, a vaccine against disease-causing low-density lipoprotein, or LDL, cholesterol can dramatically slow disease progression and maybe help avoid a trip to the cardiac catheterization lab and/or operating room, more immersions are likely ahead.
At MCG, Hedrick will also serve as director of the Cancer Immunology, Inflammation and Toleration Program at the Georgia Cancer Center. Ley also will be part of the new Transdisciplinary Research Initiative in Inflammaging and Brain Aging or TRIBA, a multimillion-dollar investment AU President Brooks Keel announced in 2021 to help address the reality that our society is aging and inflammation increases with age, quite literally affecting health from head to toe.
Both scientists were named world experts in monocyte research in 2021 by Expertscape. Ley and Munn were ranked among the top immunology scientists this year by Research.com.
Hedrick was awarded the Special Recognition Award in Vascular Biology from the American Heart Association in 2013 and chaired the Gordon Research Conference on Atherosclerosis in 2019. One of her favorite aspects of her work is mentoring young scientists, and she received the Outstanding Mentor of Women Award from the American Heart Association in 2015. Hedrick chairs the Program Project Grant Review Parent Committee of the National Heart, Lung and Blood Institute. She also is a section editor for The Journal of Immunology.
Ley is a longtime member of the American Heart Association Council on Basic Cardiovascular Science and Arteriosclerosis, Thrombosis and Vascular Biology Council and the AHA honored him with a Distinguished Scientist Award in 2016. He was honored with the Landis Award, the highest award of the Microcirculatory Society in 2017 and received a Pioneers in Cardiology Lifetime Achievement Award from the University Heart Center, Graz, Austria in 2020. He chaired the Gordon Research Conference on Atherosclerosis in 2015.
Connected
Not unlike innate and adaptive immunity, Hedrick and Ley typically work amazingly together, in life and in science. The two married in 2016.
“We complement each other in many ways,” Ley says. “Even with building this new center, I see that we have different strengths. It’s easy for her to see and meet new people and network, including with prospective recruits.” But he does enjoy talking to the public about science. Hedrick is an interesting combination of high energy and calm. Ley also appears calm but is clearly intense, so maybe they are not really so different. “He’s brilliant,” she says, “very cerebral. I can take some down time and read a cool spy novel … in his down-time he will read about climate change or politics. It’s just different levels of intensity.”
But the connection is clear. So is the tenacity. “You have to have frustration tolerance (in science),” he says. “Most things don’t work, most papers get rejected, most grants don’t get funded, so you have to have a healthy dose of stubbornness.”

————————————-
To support the Immunology Center of Georgia visit https://mcgfoundation.org/immunology-center-of-georgia/call 706-721-4001 or email philanthropy@augusta.edu