Action of Actin
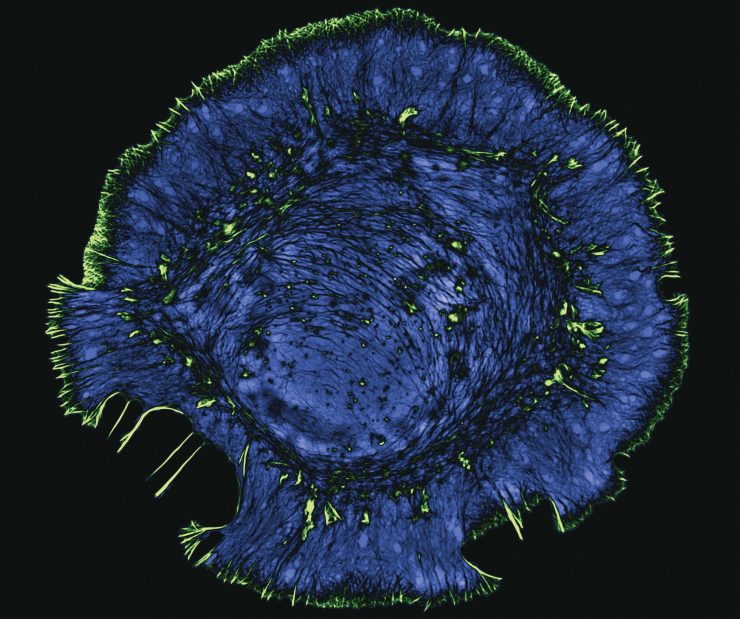
Dr. Eric Vitriol pulls up microscopy video of the actin cytoskeleton of a motor neuron, and its surface resembles the dancing edges of the sun with its solar flares.
The dynamic cytoskeleton enables a developing neuron to sense its environment, and steers it to the exceedingly precise location, where it should connect with a muscle cell, a connection which can be more than three feet away. It generates forces and gives motor neurons structural support. It allows the cells to communicate with each other and internally, to divide and to move.
“The cell is constantly sensing its surrounding, so it’s moving all the time even though it’s not changing position,” says research partner Dr. Tracy-Ann Read.
The protein actin, found in and made by essentially all cells, in its single molecule, or monomer, state is the essential building block of the linear filaments that are the scaffolding for the dynamic cytoskeleton.
Actin monomers are constantly stacking up to form filaments that organize into these elaborate, dynamic architectures in cells, then disassembling as well in orderly fashion back to single actin molecules.
Motor neurons are making filaments all the time, whether you are sedentary or a marathon runner, because like us, filaments wear out and change with age. The actin filaments Vitriol and Read study assemble in seconds and last for one to five minutes before disassembly.
The wonders of modern microscopy, with the help of fluorescent labels, enables Vitriol and Read, both cell biologists and neuroscientists, to watch it all.
“It’s like a skeleton made of Legos that you keep putting together and taking apart,” says Vitriol. “You need to see it in order to really understand what is happening.”
They have evidence that what is happening in amyotrophic lateral sclerosis, or ALS, is disruption of this essential dynamic in the motor neurons that innervate our muscles so we can move.
They are going back to the actin building blocks to better understand how it’s done right, and what can go wrong.
“This might be a common mechanism that would help us better understand ALS,” Vitriol says.
The some half-dozen known “causes” of ALS don’t have apparent common ground, Read says. But the nearly 150 genes associated with ALS all interact with the actin cytoskeleton in some way. “If you could find common mechanisms between seemingly unrelated genes then you might find something that is causing ALS,” he says.
Disabling disconnections
In ALS, motor neurons die and you lose the ability to control your muscle cells, which also eventually die, Read says. “Lou Gehrig had some of the best motor neuron connections in modern history and what happens at some point in your life, you basically enter a rapid shutdown and system failure,” says Vitriol. Problems can start with weakness, spasticity and a hard time gripping things, and progress to where you cannot walk, breathe or swallow. But the neurons we think with most typically continue to work fine. Also, somewhat ironically, along the way, some patients develop inappropriate reflexes as nerves that are still alive start making some bad connections.
There are more than a dozen different types of motor neurons, some of which die early, others later. Read and Vitriol are working to understand what drives the degeneration of motor neurons in ALS, with the long term goal of designing targeted therapeutics to intervene.
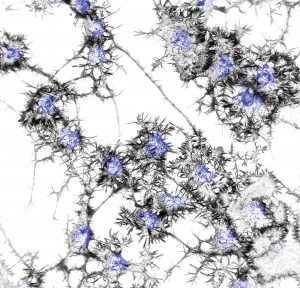
in differentiated neuronal cells.
Their focus includes, similar to those Legos, how actin molecules connect to form filaments, then later disconnect, and how that essential, ongoing process can go bad, and what happens to motor neurons when it does, which is where ALS comes in.
They began connecting these potentially important dots when they discovered that a pathway in neurons important to their development and regeneration involves novel regulation of the actin cytoskeleton, and one of the genes important for that is the actin-binding protein profilin.
They reported in the journal Current Biology last year that profilin, which is also made by motor neurons, is a master regulator of these Lego pieces, both causing them to bind to form filaments when/where needed and preventing formation when/where they aren’t. In other words, profilin controls what types of filaments get made and the structure the cell takes. They have even shown that the amount of profilin controls the types of filaments assembled.
It’s a process that is happening quickly and constantly in busy motor neurons. “If you control the supply, you control the product,” Vitriol says, and profiling apparently is in control.
About that same time another group looking for more genes associated with ALS reported that mutations in profilin caused ALS in humans and that the mutations kept profilin from its natural binding to actin. The work provided more clues that the actin cytoskeleton held clues to motor neuron degeneration in the disease.
Now they want to know if structure or the function of actin or the polymerization, or stacking, of actin molecules is part of that and if it’s a factor in other, more common types of ALS, not just the familial type caused by profilin mutations.
“We know that profilin is messed up in some forms of ALS, that the cytoskeleton is messed up in several kinds of ALS, so realistically what the profilin mutants show you is that the cytoskeleton is probably important for disease progression,” Vitriol says.
“We are trying to figure out if actin regulation, if the way actin is regulated by different proteins it interacts with or its structure, if any of that is dysregulated in ALS,” Read says. They already know that when profilin goes bad, so does actin.
Rare and precious access
A $1.86 million Maximizing Investigators’ Research Award to Vitriol from the National Institutes of Health last summer, is enabling the sort of back to basics approach that will add insight into neurodegenerative diseases like ALS as well as heart disease, cancer and inflammatory disorders.
They have looked at some of the more common ALS associated genes and found a lot of them affect the actin cytoskeleton. Gene mutations that cause the hereditary form of ALS first enabled ALS studies in the lab, and scientists watched what happened to immortalized, commercially available cell lines when the mutants were expressed. But these don’t have a lot of disease relevance, Vitriol says.
Their lab is one of the few that has been able to also culture spider-like adult motor neurons from mice. When examined at the end stages of ALS, they have seen the actin cytoskeleton is dramatically different, more evidence that actin is messed up in ALS, but still not enough, they say.
“ALS can run in families but 90% are spontaneous and you can’t recreate that in a mouse colony,” Vitriol says. Working with human samples can enable that exploration and is much of what brought the two to the Medical College of Georgia early in 2021 from the University of Florida.
“If we really want to treat ALS properly, we need to know what the disease really is,” says Dr. Michael Rivner, medical director of the ALS Center at MCG’s affiliated AU Health System, an ALS Association certified treatment center of excellence in Augusta. Rivner has been taking care of patients with ALS about 30 years and notes that his slide on the causes of ALS in his also now 30-year-old, although regularly updated, ALS presentation really has not changed much.
The treatment options have not either, with two approved drugs, riluzole and edaravone providing some slowing down of the disease, says Dr. Ben Barnes, neuromuscular neurologist who joined Rivner at MCG late last summer.
“The bottom line comes back to, we don’t know the cause(s), which is why besides having a clinical research component, we need to have a strong basic science component,” Rivner says. So he and Barnes worked hard with Department of Neuroscience and Regenerative Medicine chair Dr. Xin-Yun Lu and others to recruit Vitriol and Read.
At MCG, the new recruits have what they consider “rare and precious” access to patient tissue and to the physicians who care for patients.
To develop studies of the more common, spontaneous ALS, the scientists now will be able to use the skin or blood of patients to make human motor neurons, as well as muscle samples to look at their muscle cells. They will even use these valuable human materials to rebuild the point of interaction between motor neuron and muscle cell, a fine point called the neuromuscular junction, which enables us to move and which the cytoskeleton enables.
Vitriol says that connection will happen if you just co-culture this natural cell pairing. While in ALS, dying motor neurons retract their connections with their partner, Read says.
While there is no definitive test for ALS, part of the evaluation includes a nerve biopsy to ensure it is a nerve problem, Barnes says. He and Rivner are confident most of their patients will also readily agree to a small, safe punch biopsy to provide the other tissue needed to move science forward. Their families likely will happily supply the normal control samples needed.
The always-pragmatic Rivner says he knows he and Barnes are making patients’ lives better through the care they offer at the bimonthly ALS clinic. To enhance offerings they are recruiting a third subspecialist and seeking center status for the ALS clinic at Atrium Health Navicent in Macon, Georgia, which Rivner also directs. He and Barnes, along with Brandy Quarles, research operations coordinator, also conduct clinical trials, currently exploring the potential of three new immune modulators that should reduce damaging inflammation around motor neurons.
But Rivner readily admits he wants the tools to do more. “It’s hard to tell patients the diagnosis; it’s hard to see the disease spread.”
Back Story
Read was born in the United Kingdom, moved to Norway while still a child — dad was English, mom Norwegian — a country which was beautiful and which she loved. Her early plan was to become a lawyer, but she was pulled by the dynamics of science, particularly neuroscience. “I think what I like most about science is that it is forever changing. Nothing becomes stagnant,” she says. “You learn something then you learn something else then you have to modify your first opinion,” she says. It was the pursuit of science that took her from a country of 5 million to the United States after she earned her PhD at the Department of Anatomy and Cell Biology at the University of Bergen. She completed her postdoctoral training in the Department of Pathology at Duke University and Department of Neurosurgery at Johns Hopkins University, before becoming a senior research associate at Duke University and the University of Bergen. She was on the faculty of Emory University School of Medicine and the University of Florida College of Medicine before coming to MCG in early 2021. She has served as director of the Pediatric Neuro-oncology Laboratory at Children’s Healthcare of Atlanta and as a member of Emory’s Winship Cancer Institute.
Vitriol earned his PhD in cell and developmental biology at the University of North Carolina at Chapel Hill, did his postdoctoral studies at Emory University and joined the University of Florida College of Medicine in 2014, where he was a member of the Center for Translational Research In Neurodegenerative Disease and McKnight Brain Institute. Vitriol, who fortunately does not appear to live up to the literal definition of his name, didn’t know what he wanted to do until he enrolled at a community college in south Florida and met science teacher Dr. Charlie Ray. Dr. Ray taught every class — zoology, organic chemistry, introduction to biology — without notes, had two PhDs and gave tests that were impossible to finish on time but he took the time to give his students, who were willing to work hard, the opportunity to keep working on the tests until they got them right. Like Read, (and Ray’s testing techniques) Vitriol began to see the magic of science’s fluidity. Vitriol would transfer to the University of North Carolina to finish his undergraduate degree in biology (with honors) but is certain that if he had never met Dr. Ray, there is no telling what he would have become. He did like music and saw the rock band Phish 103 times, but there was the small problem that he had no musical talent. When he met Dr. Kerry Bloom, now chair of biology at UNC, and then an outstanding cell biologist and microscopist, the deal was sealed on research, on visualizing and better understanding the incredible, complex dynamic of even a single living cell as they tagged a chromosome, made a break in the DNA, then watched what it did to the chromosome’s integrity. When he was doing his last interview for graduate school, Dr. Richard Cheney at UNC Chapel Hill showed him a movie of a cytoskeletal protein moving up and down the cytoskeleton. It looked like fireworks to Vitriol and imploded his plan to study DNA damage and repair and cancer at Duke University.
The couple in the lab and in life met while he was in graduate school and she was doing her postdoc in North Carolina.
Today he still finds the cytoskeleton a beautiful, and unbelievably dynamic structure and he, Read and their research team are helping define how it is far more than a cell framework.